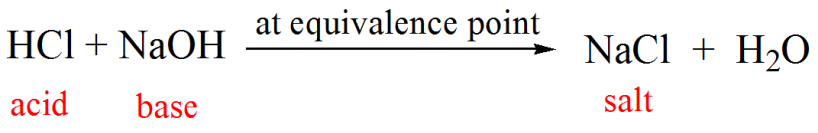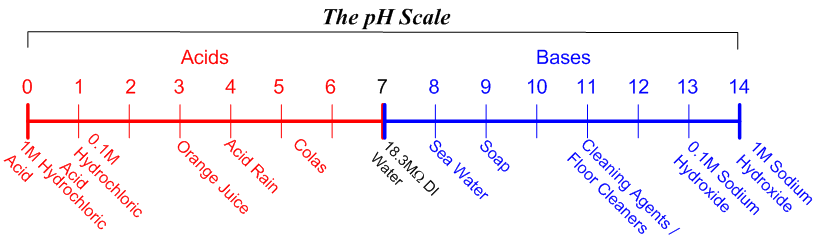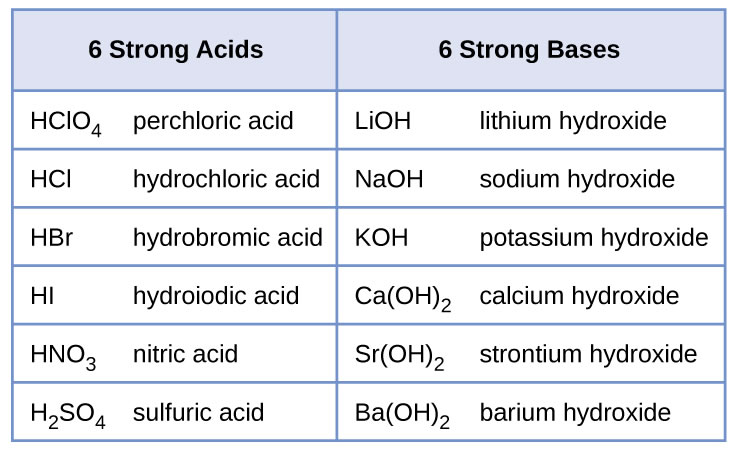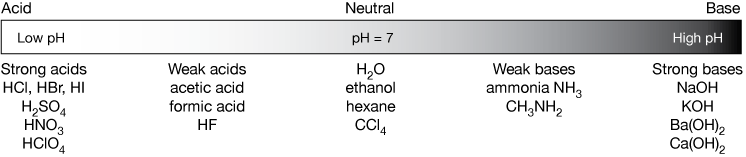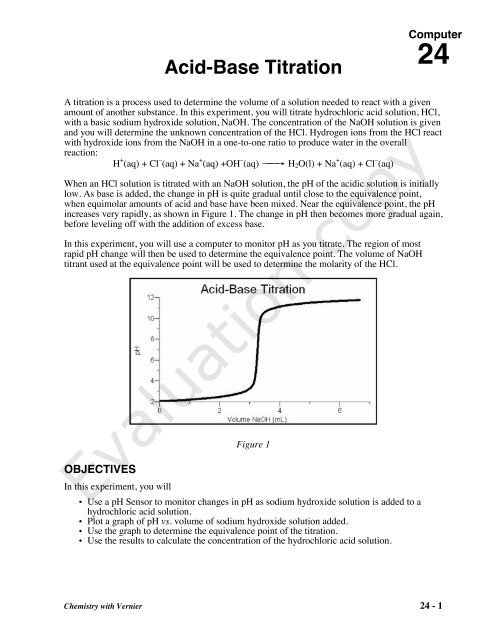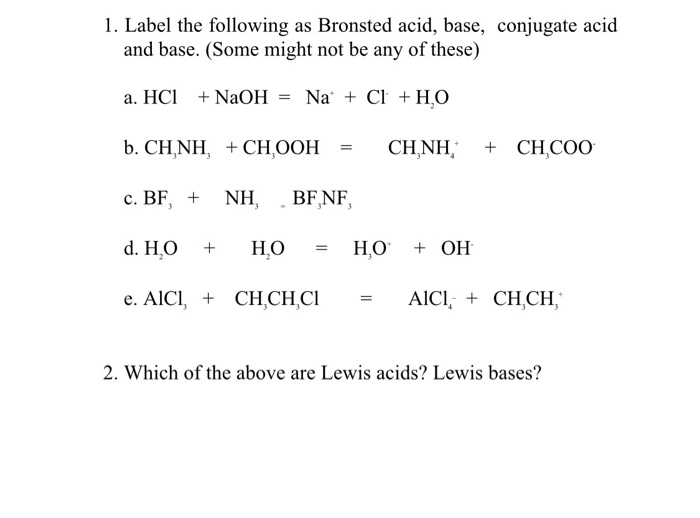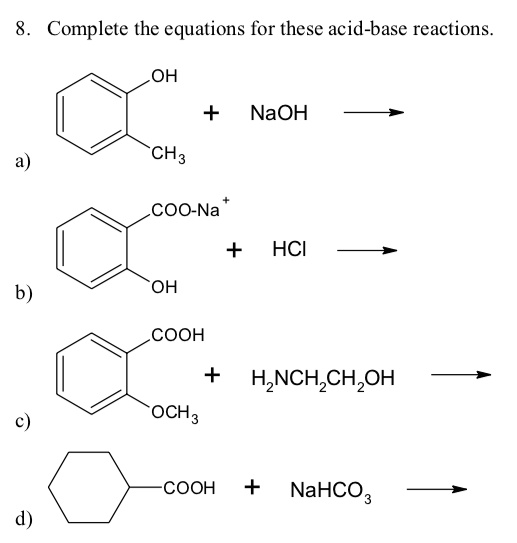
SOLVED: Complete the equations for these acid-base reactions. OH NaOH CH3 COO-Na HCI b) OH COOH H2NCHZCHZOH COOH NaHCO OCHg

RICCA CHEMICAL COMPANY - Sodium Hydroxide is a strong base in terms of chemical ionization and solutions of it can be assayed using a strong acid, such as Hydrochloric Acid or Sulfuric
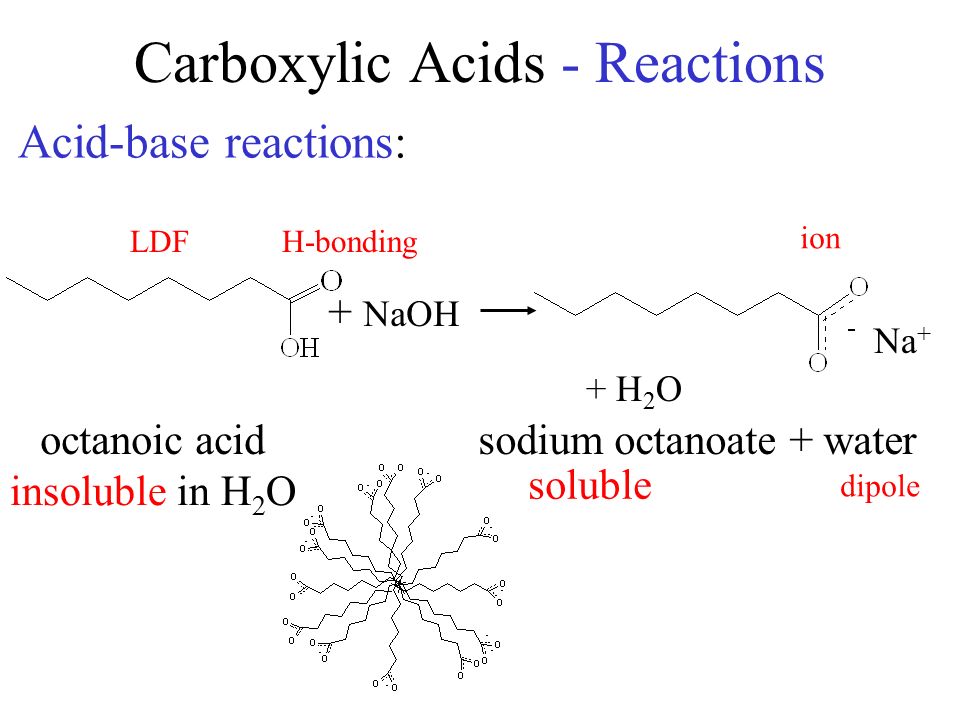
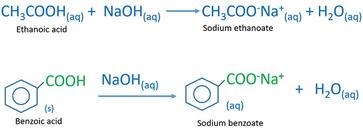
Carboxylic Acids - Reactions Acid-base reactions: octanoic acid + NaOH Na + + H 2 O sodium octanoate + water insoluble in H 2 O soluble H-bondingLDF ion. - ppt download

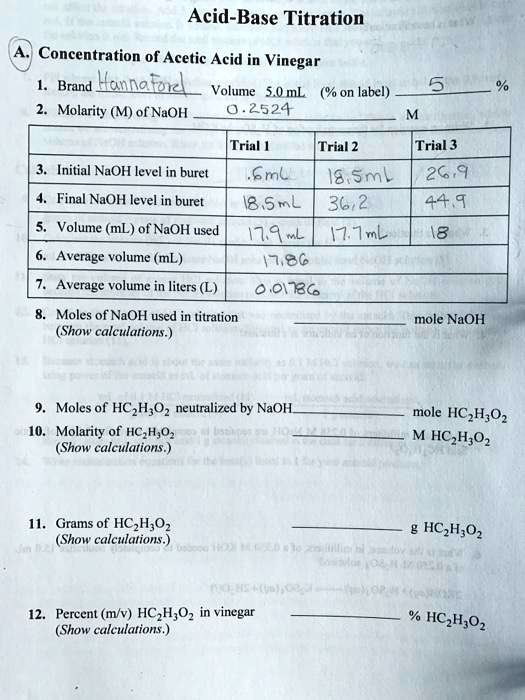
SOLVED: LA REPORT SHEET EXPERIMENT Titration of Acids and Bases 20 A. Analysis of an Unknown Acid Trial 3 Trial 2 Trial Mass of bottle unknown Mass of bottle Mass of unknown